CAR T-Cell Therapy for Mesothelioma
CAR T-cell treatment is an emerging immunotherapy for cancer patients. It takes a patient’s immune cells and modifies them in a lab to better recognize and kill cancer cells. CAR T cells have worked quite well for blood cancers. Clinical trials are studying the effects of this treatment against mesothelioma.
Learn About Immunotherapy for Mesothelioma in Our Free Guide
What Is CAR T-Cell Therapy?
CAR T-cell therapy is a form of immunotherapy being tested in clinical trials. The therapy has been approved by the U.S. Food and Drug Administration (FDA) to treat certain blood cancers. CAR T-cell therapy is being tested in clinical trials for mesothelioma and other solid tumors.
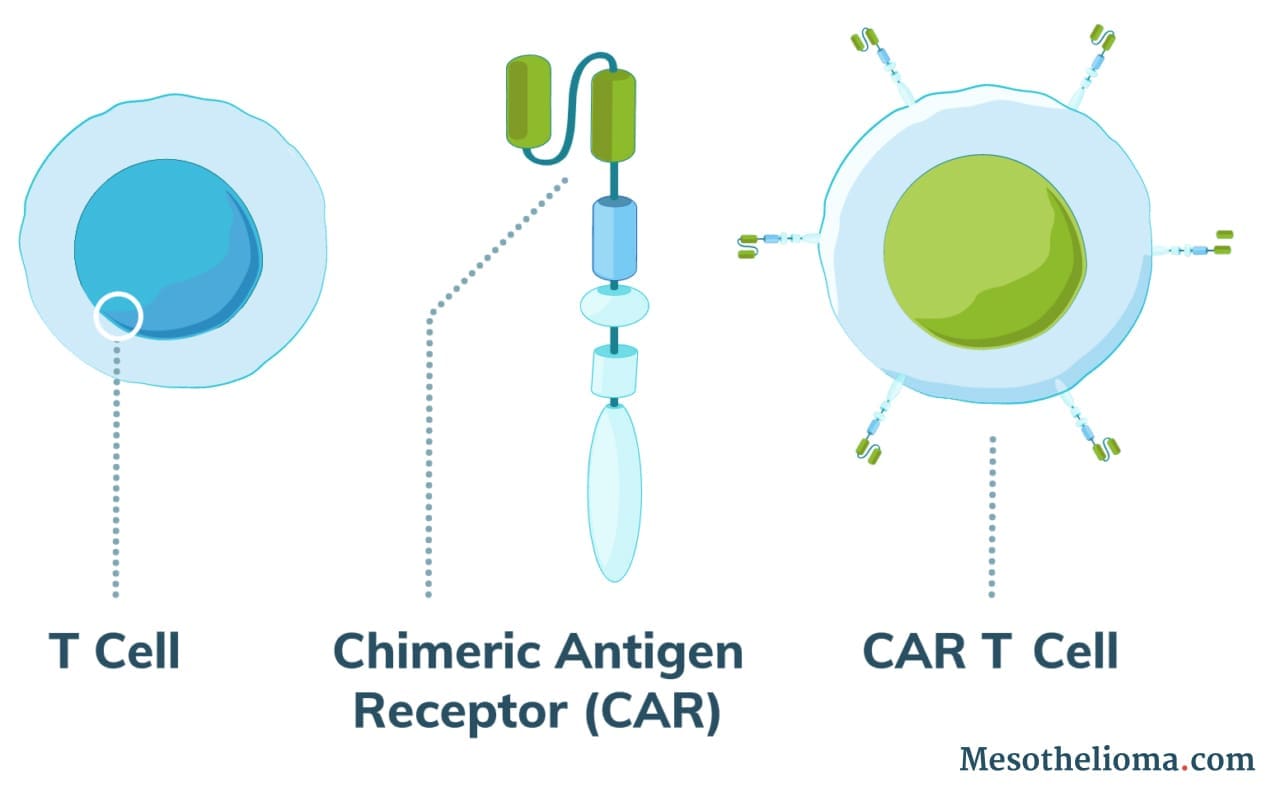
Normal T cells (white blood cells) protect the body against disease and can recognize cancer cells. However, in most cases, cancer cells evade the immune system by tricking T cells into not attacking them. Using CAR T-cell therapy, doctors can engineer T cells to identify and attack cancer.
How Does CAR T-Cell Therapy Work?
CAR T-cell therapy works by adding altered T cells to a patient’s body to fight cancer. Doctors use a process called adoptive cell therapy (ACT) to collect a patient’s cells and modify them.
The altered T cells produce receptor proteins called chimeric antigen receptors (CAR). As a result, modified cells are CAR T cells.
CAR T cells can recognize a protein specific to cancer cells. These proteins are called tumor-associated antigens (TAAs). Researchers have tried to identify types of TAAs that are very common on the cell surface of tumor cells but uncommon on healthy cells.
When CAR T cells can identify TAAs, they can target and attack cancer cells. In order to treat mesothelioma, doctors will target TAAs commonly found on the mesothelioma cancer cells’ surface.
Researchers have identified a protein called mesothelin as a good TAA for mesothelioma. It appears on the outside surface of cancer cells. And studies show CAR T cells aimed at mesothelin can attack and kill mesothelioma cells.
The CAR T-Cell Therapy Process
During treatment, the CAR T-cell therapy process consists of:
- Acquiring a patient’s T cells
- Modifying the T cells in a laboratory to have CAR receptors that recognize TAAs specific to cancer cells
- Growing and multiplying a patient’s modified CAR T cells
- Administering the CAR T cells intravenously or intrapleurally depending on the patient’s case
In some cases, the treatment can be completed with a single infusion.
The new receptor on the CAR T cells is designed to recognize cancer cells. Once the CAR T cells encounter cancer, they can attack and kill the cancer cell carrying the protein.
As a result, CAR T cells boost the immune system to fight cancer cells.
The CAR T cells may continue to fight cancer cells years after treatment. In some cases, if the patient has a relapse, the CAR T cells may still be able to find and destroy new cancer cells.
CAR T-cell treatment is most commonly recommended for patients not responding well to other forms of cancer treatment. Before undergoing CAR T-cell treatment, mesothelioma patients will need to be accepted into a clinical trial.
Does CAR T-Cell Therapy Improve Mesothelioma Prognosis?
Only a few clinical trials have looked at CAR T cells for mesothelioma. In those studies, survival results varied. Some mesothelioma patients lived only a few months, others lived years or longer. This means it’s too early to tell if or how much this treatment can improve survival. But early results show some promise.
CAR T Cells With a Checkpoint Inhibitor Seem to Boost Survival
In a recent study, doctors looked at combining CAR T cells with a checkpoint inhibitor. They first treated pleural mesothelioma patients with CAR T cells. Several weeks later, the patients received Keytruda® (pembrolizumab). All study patients had undergone treatment before joining the trial.
Half of the study patients survived longer than 2 years (the median survival). In other studies of second-line treatments, the median survival was less than a year. So CAR T cells with Keytruda may improve life expectancy if it becomes a standard treatment in the future.
Questions About Mesothelioma Immunotherapy Treatment? Ask experienced mesothelioma advocate Jennifer Lucarelli Ask a QuestionCAR T-Cell Research for Treating Mesothelioma
CAR T-cell therapy initially came about as a way to treat blood cancers. But recent clinical trials have studied it for treating solid tumors, including mesothelioma and lung cancer. These studies found that solid tumors present some new challenges for CAR T cells.
- Cancer cell variety: Solid tumors contain different types of cells that don’t all look the same. So CAR T cells aren’t able to recognize (and kill) all the cells in this type of tumor.
- Dense tumor structure: CAR T cells can only go so far into a tumor. This means they can’t reach cancer cells past the outer layers of the tumor.
- Suppressive environment: Cancer cells send out signals that stop CAR T cells from working. So CAR T-cell therapies may initially work but quickly fizzle out.
Many ongoing clinical trials are working to address these challenges. Several approaches have been suggested, including combining CAR T cells with other therapies. Combining therapies often improves patient survival and other outcomes. Researchers are also searching for new TAA targets to make it easier for CAR T cells to recognize cancer cells.
For example, the National Cancer Institute launched a new CAR-T cell trial in July 2025. It will investigate treating mesothelioma and other solid tumors with CAR T cells. Other trials are also working on different approaches to CAR-T cell therapy.
Off-the-Shelf CAR T Cells for Mesothelioma and Other Cancers
In this early-phase trial, researchers are testing a new type of CAR T cell. Instead of using a patient’s own cells, they will use donor cells. This approach is aimed at making the process faster and easier for patients. Researchers will use this treatment for several types of cancer, including mesothelioma. They expect the study to conclude sometime in 2030.
CAR T Cells With Next-Gen Recognition
In general, CAR T cells recognize and attack cells that have the targeted TAA. But even well-selected TAAs can be present on healthy cells. This means CAR T cells can cause side effects by attacking normal tissue.
This early-phase trial is looking to address that problem with an improved version of CAR T cells. The upgrade is designed to stop them from attacking healthy cells. Scientists have referred to this addition as a safety switch designed to protect normal tissue. The study will accept patients with several types of cancer, including mesothelioma. Researchers expect it to conclude sometime in 2029.
Side Effects of CAR T-Cell Therapy
CAR T-cell therapy has some side effects and risks. But several clinical trials are actively researching ways to reduce these complications.
The following side effects may occur during or after CAR T-cell therapy:
- CAR T Cell-Related Encephalopathy Syndrome (CRES)
- Confusion
- Cytokine release syndrome (CRS)
- Difficulty breathing
- Dizziness
- Fever
- Immune reactions (anaphylaxis)
- Joint or muscle aches
- Low blood pressure
- Nausea
- Seizures
The most common side effect associated with CAR T-cell therapy is Cytokine Release Syndrome (CRS). CRS may cause mild flu-like symptoms, low blood pressure and increased heart rate. In many cases, it can be managed with other treatments.
Due to limited research, it’s unclear how often mesothelioma patients may experience side effects. Researchers will continue to document how mesothelioma patients are affected by this form of immunotherapy.
Is CAR T-Cell Therapy Successful in Treating Mesothelioma?
CAR T-cell therapy may be a viable treatment option for mesothelioma patients in the future. However, the treatment is still being researched and tested.
Although CAR T-cell therapy has been approved to treat other forms of cancer, there is not enough data to determine its success against mesothelioma. Researchers will continue to test the treatment with the goal of improving survival rate for mesothelioma patients.
Common Questions About CAR T Cells
Where is CAR T-cell therapy available?
What are the most recent developments in CAR T-cell therapy?
CAR T cell therapy is an area of active research, so developments are happening in many arenas:
- November 2024: A new type of CAR T-cell earned FDA approval for treating a specific form of leukemia.
- June 2025: The FDA removed some requirements from two already approved CAR T-cell therapies. This move aims to make CAR T cells more readily available to qualified patients.
Which cancers are treated with CAR T-cell therapy?
Sources
Cakiroglu E, Eris S, Oz O, Karakülah G, Senturk S. Genome-wide CRISPR screen identifies BUB1 kinase as a druggable vulnerability in malignant pleural mesothelioma. Cell Death Dis. 2025 Apr 3;16(1):241.
Calabrò L, Bronte G, Grosso F, Cerbone L, Delmonte A, Nicolini F, et al. Immunotherapy of mesothelioma: the evolving change of a long-standing therapeutic dream. Front Immunol. 2023;14:1333661.
Cao L, Liu Y, Lin G. Strategies for altering delivery technologies to optimize car therapy. Int J Mol Sci. 2025 Mar 30;26(7):3206.
Cedres S, Valdivia A, Iranzo P, Callejo A, Pardo N, Navarro A, et al. Current state-of-the-art therapy for malignant pleural mesothelioma and future options centered on immunotherapy. Cancers (Basel). 2023 Dec 10;15(24):5787.
Erickson SM, Manning BM, Kumar A, Patel MR. Engineered cellular therapies for the treatment of thoracic cancers. Cancers (Basel). 2024 Dec 26;17(1):35.
Garitaonaindia Y, Martínez-Cutillas M, Uribarren M, Redondo I, Calvo V, Serna-Blasco R, et al. Adoptive cell therapies in thoracic malignancies: a comprehensive review. Clin Transl Oncol. 2025 Jan 9;
Suraya R, Nagano T, Tachihara M. Recent advances in mesothelioma treatment: immunotherapy, advanced cell therapy, and other innovative therapeutic modalities. Cancers (Basel). 2025 Feb 18;17(4):694.
Umeyama Y, Taniguchi H, Gyotoku H, Senju H, Tomono H, Takemoto S, et al. Three distinct mechanisms underlying human γδ T cell-mediated cytotoxicity against malignant pleural mesothelioma. Front Immunol. 2023;14:1058838.
Xu T, Tian T, Wang C, Chen X, Zuo X, Zhou H, et al. Efficacy and safety of novel multiple-chain DAP-CAR-T cells targeting mesothelin in ovarian cancer and mesothelioma: a single-arm, open-label and first-in-human study. Genome Med. 2024 Nov 15;16(1):133.
Yang Y, Vedvyas Y, Alcaina Y, Trumper SJ, Babu DS, Min IM, et al. Affinity-tuned mesothelin CAR T cells demonstrate enhanced targeting specificity and reduced off-tumor toxicity. JCI Insight. 2024 Nov 22;9(22):e186268.
Zhao S, Zhao H, Yang W, Zhang L. The next generation of immunotherapies for lung cancers. Nat Rev Clin Oncol. 2025 Aug;22(8):592–616.
Free Mesothelioma Treatment Guide


Dr. Francis Perry Wilson is the Director of the Clinical and Translational Research Accelerator at the Yale University School of Medicine. He specializes in nephrology and clinical research.

Katy Moncivais, Ph.D., has more than 15 years of experience as a medical communicator. As the Medical Editor at Mesothelioma.com, she ensures our pages and posts present accurate, helpful information.


