What Is CAR T-Cell Therapy?
CAR T-cell therapy is a form of immunotherapy being tested in clinical trials. The therapy has been approved by the U.S. Food and Drug Administration (FDA) to treat certain blood cancers. CAR T-cell therapy is being tested in clinical trials for mesothelioma and other solid tumors.
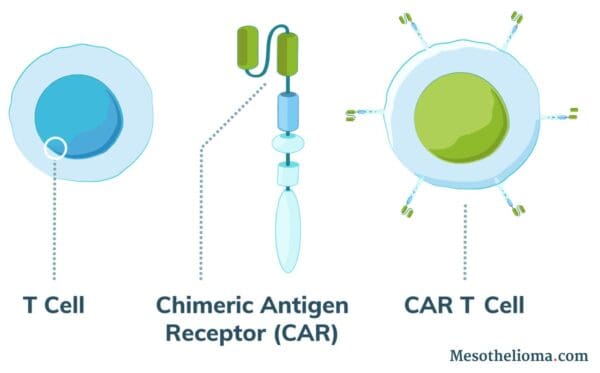
Normal T cells (white blood cells) protect the body against disease and can recognize cancer cells. However, in most cases, cancer cells evade the immune system by tricking T cells into not attacking them. Using CAR T-cell therapy, doctors can engineer T cells to identify and attack cancer.
How Does CAR T-Cell Therapy Work?
CAR T-cell therapy treatment works by adding altered T cells to a patient’s body to fight cancer. Doctors use a process called adoptive cell therapy (ACT) to collect a patient’s cells and modify them.
The altered T cells produce receptor proteins called chimeric antigen receptors (CAR). As a result, modified cells are CAR T cells.
CAR T cells can recognize a protein specific to cancer cells. These proteins are called tumor-associated antigens (TAAs). Researchers have tried to identify types of TAAs that are very common on the cell surface of tumor cells but uncommon on healthy cells.
When CAR T cells can identify TAAs, they can target and attack cancer cells. In order to treat mesothelioma, doctors will target TAAs commonly found on the mesothelioma cancer cells’ surface.
The CAR T-Cell Treatment Process
During treatment, the CAR T-cell therapy process consists of:
- Acquiring a patient’s T cells
- Modifying the T cells in a laboratory to have CAR receptors that recognize TAAs specific to cancer cells
- Growing and multiplying a patient’s modified CAR T cells
- Administering the CAR T cells intravenously or intrapleurally depending on the patient’s case
In some cases, the treatment can be completed with a single infusion.
The new receptor on the CAR T cells is designed to recognize cancer cells. Once the CAR T cells encounter cancer, they can attack and kill the cancer cell carrying the protein.
As a result, the CAR T cells boost the immune system to fight cancer cells.
The CAR T cells may continue to fight cancer cells years after treatment. In some cases, if the patient has a relapse, the CAR T cells may still be able to find and destroy new cancer cells.
CAR T-cell treatment is most commonly recommended for patients not responding well to other forms of cancer treatment. Before undergoing CAR T-cell treatment, mesothelioma patients will need to be accepted into a clinical trial.
Resources for Mesothelioma Patients
How CAR T-Cell Therapy May Help Mesothelioma Patients
Recent clinical trials have studied the value of CAR T-cell therapy for solid tumors, including mesothelioma and lung cancer.
CAR T-cell immunotherapy may be an option for mesothelioma patients who are ineligible for cytoreductive surgery, chemotherapy and other traditional mesothelioma treatments.
At this time, mesothelioma patients can only seek CAR T-cell therapy through clinical trials. In order to participate in a clinical trial, patients must apply and meet the criteria for the study.
Mesothelioma Clinical Trials Testing CAR T-Cell Therapy
CAR T-cell immunotherapy for mesothelioma is currently being tested in limited clinical trials. In some trials, mesothelioma doctors have seen some promising patient responses to CAR T-cell therapy.
Researchers from the Memorial Sloan Kettering Cancer Center in New York City have tested this emerging treatment for mesothelioma. Two recent clinical trials tested the efficacy of CAR T-cell therapy on a small number of pleural mesothelioma patients.
Researchers Use CAR T Cells to Target Proteins on Mesothelioma Cells
From 2012 to 2019, researchers studied the efficacy of CAR T cells targeting FAP. For some patients, they combined these FAP-targeting CAR T cells with a PD-1 blocking antibody.
In this study, researchers tested a different type of CAR T cell against mesothelioma. All patients underwent an intrapleural infusion of CAR T cells targeting FAP, a TAA commonly found on mesothelioma cancer cells.
One group of patients also received the PD-1 blocking antibody. PD-1 is a protein found on immune cells. Blocking this protein makes it easier for the CAR T cells to attack mesothelioma cells.
Specifically, patients in the study underwent:
- Intrapleural infusion of CAR T cells targeting FAP (2 patients)
- Intrapleural infusion of CAR T cells targeting FAP + anti-PD-1 therapy (1 patient)
At a median follow-up of 18 months, 2 of 3 patients are still alive. The patients also did not experience many side effects. However, the size of this study is too small to draw conclusions about the effectiveness of the therapy.
Ongoing Clinical Trial Finds About 50% of Patients Experience Tumor Response
In an ongoing mesothelioma clinical trial, researchers are testing mesothelin-targeted CAR T cells in combination with an anti-PD-1 agent.
A group of 18 mesothelioma patients underwent an intrapleural infusion CAR T cells to target TAAs commonly found on mesothelioma cancer cells.
- 14 patients were given CAR T cells targeting mesothelin with anti-PD-1 therapy
- 4 patients were given CAR T cells targeting mesothelin
The current reported results only include the 14 patients who received CAR T cells targeting mesothelin with anti-PD-1 therapy.
According to the published results, tumors shrunk or disappeared in approximately 50% of patients. Patient results include:
- Complete Metabolic Response (tumors are gone): 2 patients
- Partial Response (tumors shrunk but have not disappeared): 5 patients
- Stable Disease (tumors did not grow or shrink, and there are no new tumors): 4 patients
The results appear to be promising, but the size of the group is too small to draw conclusions for treating mesothelioma. This study is ongoing and researchers will continue to publish results.
In the future, CAR T-cell therapy may be a good option for mesothelioma patients who don’t qualify for other forms of treatment. Doctors continue to test the effectiveness and side effects of the therapy for mesothelioma.
Side Effects of CAR T-Cell Therapy
There are side effects and risks associated with CAR T-cell therapy. As research and clinical trials continue, doctors will continue working towards mitigating CAR T-cell therapy side effects.
The following side effects may occur during or after CAR T-cell therapy:
The most common side effect associated with CAR T-cell therapy is Cytokine Release Syndrome (CRS). CRS may cause mild flu-like symptoms, low blood pressure, increased heart rate and heart failure. In many cases, it can be managed with other treatments.
Due to limited research, it’s unclear how often mesothelioma patients may experience side effects. Researchers will continue to document how mesothelioma patients are affected by this form of immunotherapy.
Is CAR T-Cell Therapy Successful in Treating Mesothelioma?
CAR T-cell therapy may be a viable treatment option for mesothelioma patients in the future. However, the treatment is still being researched and tested.
Although CAR T-cell therapy has been approved to treat other forms of cancer, there is not enough data to determine its success against mesothelioma. Researchers will continue to test the treatment with the goal of improving survival rate for mesothelioma patients.