PIPAC for Mesothelioma
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a method of administering chemotherapy. This technique may help patients become eligible for surgery plus a heated chemotherapy called HIPEC. Surgery and HIPEC may help extend life expectancy for peritoneal mesothelioma patients.
Request a Free 2026 Mesothelioma Guide Free 2026 Mesothelioma Guide
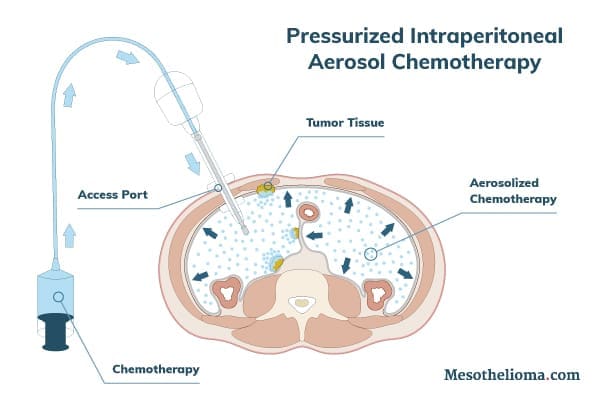
What Is PIPAC Treatment?
PIPAC stands for pressurized intraperitoneal aerosol chemotherapy. It is an emerging form of mesothelioma treatment. PIPAC uses high pressure to push chemotherapy drugs into tumor tissue. This approach may make it more effective than traditional chemotherapy.
Clinical trials are exploring PIPAC’s potential benefits for peritoneal mesothelioma patients. PIPAC treatment may help patients ineligible for cytoreductive surgery (CRS) become eligible. Early research also suggests it may help improve peritoneal mesothelioma symptoms.
Cytoreductive surgery (CRS) is also known as “debulking.” It is a surgical procedure in which doctors attempt to remove as much of the visible tumor as possible.
PIPAC uses chemotherapy drugs to target tumor cells in the abdomen. The PIPAC procedure applies the drugs as a high-pressure aerosol. This allows the chemotherapy drugs to reach more tumor cells than standard chemotherapy methods.
Mesothelioma patients interested in PIPAC can discuss this procedure with their doctor. A mesothelioma doctor can help determine if a patient may be eligible for a PIPAC clinical trial.
PIPAC vs. HIPEC
PIPAC and heated intraperitoneal chemotherapy (HIPEC) are methods of administering chemotherapy to the abdominal cavity. Both methods treat peritoneal mesothelioma. HIPEC is a more common mesothelioma treatment than PIPAC. But PIPAC has qualities that may make it another useful tool in the mesothelioma treatment toolbelt. For example, it may help patients ineligible for CRS become eligible.
Both PIPAC and HIPEC administer chemotherapy drugs directly into the abdomen. PIPAC applies chemotherapy as a pressurized aerosol. HIPEC applies chemotherapy as a heated wash and is often combined with surgery. HIPEC plus surgery has proven effective in improving life expectancy for peritoneal mesothelioma patients.
Patients who undergo HIPEC procedures typically stay at the hospital for 10 to 12 days. Those who undergo PIPAC may be discharged on the same day as the procedure. Doctors will continue to monitor recovery after the patient is discharged.
Clinicians may use PIPAC as a step toward CRS plus HIPEC. For example, abdominal surgery may be too risky if a tumor is too large. PIPAC may help shrink tumors enough to make surgery plus HIPEC safer for patients.
PIPAC may also be an option for patients who are not yet healthy enough for surgery plus HIPEC. Surgery is taxing on the body and may require physical preparation in the weeks before the procedure. The surgery plus HIPEC procedure itself may last 8 – 14 hours. PIPAC procedures last about two hours and generally do not need special preparation.
How Does PIPAC Treat Mesothelioma?
PIPAC cancer treatment targets the abdomen, where peritoneal mesothelioma occurs. This targeted application allows patients to receive a high concentration of chemotherapy. The pressure of the aerosolized drug can also enhance chemotherapy’s tumor cell-killing effect.
By turning the chemotherapy drug into a pressurized aerosol, PIPAC can reach more cancer cells than other methods. When it comes to its mesothelioma cancer-targeting uses, PIPAC’s potential benefits include:
- Evenly applying chemotherapy: The pressurized aerosol evenly distributes the chemotherapy throughout the abdomen.
- Pushing chemotherapy farther into tumor tissue: Tumors may have natural resistance to the entry of fluids like regular chemotherapy. The pressure of the PIPAC application helps push chemotherapy into tumor tissue. This may help it penetrate deeper than traditional fluid chemotherapy solutions.

PIPAC Procedure
The PIPAC procedure consists of four steps: preparation, administration, port removal and recovery. Patients undergoing PIPAC receive general anesthesia. This means the patient is asleep for the first three steps. The procedure lasts about two hours.
PIPAC is a laparoscopic procedure. This means doctors perform it through one or two small punctures, also called access ports, in the patient’s abdomen.
The PIPAC procedure may follow these general steps:
- Preparation: Doctors first create the access port(s) for the procedure. They then use the port(s) to inflate the peritoneum (the membrane lining the abdomen). They may also collect tumor biopsies and/or remove accumulated fluid. Finally, the doctors insert the device that applies pressurized chemotherapy into an access port.
- Administration: The pressurized, aerosolized chemotherapy is administered within the peritoneum for about 30 minutes. The excess chemotherapy gas is then flushed out through a waste management system.
- Port removal: Doctors remove all devices from a patient’s abdomen and close incisions.
- Recovery: Healthcare providers move the patient to a recovery room. The team monitors the patient until they are ready for discharge.
PIPAC treatment may involve different types of chemotherapy drugs. There is currently no standard drug or drug combination for PIPAC for mesothelioma. In one study, patients with peritoneal cancer received a combination of cisplatin and doxorubicin. Future clinical studies may explore different drugs or drug combinations for mesothelioma patients.
What to Expect After the PIPAC Procedure
After a PIPAC procedure, a patient may or may not have a hospital stay. Patients who do stay in the hospital may be there for one or two days.
At least one study concluded that PIPAC can be performed in an outpatient setting. The study outlines the conditions for a patient being discharged on the day of surgery:
- Adequate pain relief
- Free mobility
- No anxiety or discomfort caused by leaving the hospital
- No urination problems
Patients may receive several rounds of PIPAC treatment every six to eight weeks. There is currently no standard number of PIPAC treatment cycles for mesothelioma patients. In one study, peritoneal mesothelioma patients received between three to nine cycles of PIPAC. Another clinical trial plans to administer four cycles of PIPAC treatment. A patient’s care team may determine the appropriate number of cycles based on an individual’s case.
A mesothelioma patient can talk to their healthcare team about what to expect after PIPAC treatment. Members of a patient’s care team can also help with recovery.
Benefits of PIPAC for Mesothelioma
Peritoneal mesothelioma patients may benefit from PIPAC. It is a newer procedure, but early PIPAC results point to several potential advantages. For example, some patients became eligible for surgery after PIPAC treatment. Surgery, especially if combined with HIPEC, may improve mesothelioma prognosis. Some studies show 10-year survival rates of 45% for mesothelioma patients who receive surgery plus HIPEC.
PIPAC may also help improve mesothelioma symptoms. In one study, PIPAC improved symptoms for 32% of patients. It also helped control fluid buildup, known as peritoneal effusion, in 46% of patients. Peritoneal effusion may cause various symptoms, such as abdominal pain.
Other benefits of PIPAC include:
- High concentration of chemotherapy: PIPAC increases the amount of chemotherapy in tumor tissue. This can help boost the cancer-fighting ability of chemotherapy drugs. PIPAC does this without causing a high blood concentration of the drug. This may lead to less organ damage than systemic chemotherapy.
- Gastrointestinal stability: In general, gastrointestinal symptoms do not get worse with PIPAC. Gastrointestinal symptoms include nausea, vomiting, constipation, diarrhea and anorexia.
- Low toxicity: PIPAC does not appear to cause significant liver or kidney damage. This suggests that it may be safe to administer multiple cycles of PIPAC. In one study, patients who received nine cycles of PIPAC did not report any significant kidney damage.
Peritoneal mesothelioma patients interested in PIPAC should discuss the procedure with their doctor. Mesothelioma doctors can explain the potential benefits and side effects of PIPAC.
Questions About PIPAC Treatment for Mesothelioma? Ask experienced mesothelioma advocate Jennifer Lucarelli Ask a QuestionPIPAC Side Effects
Like other forms of mesothelioma cancer treatment, PIPAC may cause side effects. But it may cause fewer side effects than traditional, systemic chemotherapy. Some PIPAC patients have had the procedure more than once without significant negative effects on their quality of life.
According to one medical center, possible PIPAC side effects include:
- General discomfort
- Mild abdominal pain
- Slight nausea
Catheter-linked complications, such as infection, may also be possible.
Common systemic chemotherapy side effects may be less likely to occur with PIPAC. For instance, PIPAC patients do not typically report hair loss or worsening gastrointestinal symptoms.
Patients should talk to their care team about any side effects they experience. Their care team may be able to help manage treatment side effects.
PIPAC Clinical Trials
Research into PIPAC for mesothelioma and other cancers is ongoing. Results from clinical trials may help improve treatment for peritoneal mesothelioma. Patients who wish to join a PIPAC clinical trial can discuss eligibility with their doctor.
Early research suggests that PIPAC may be a useful part of a mesothelioma treatment plan. A recent study looked at the benefits of PIPAC in peritoneal mesothelioma patients. Study patients received PIPAC in combination with systemic chemotherapy. Researchers concluded that PIPAC is a workable treatment to try to shrink tumors and help patients qualify for surgery.
After receiving PIPAC, some study patients became eligible for and underwent surgery. These patients saw significant periods of progression-free survival. Also, almost one-third of study participants reported improvement of symptoms.
Another clinical trial is looking at using PIPAC as a potential first-line treatment. Its completion is estimated for the end of 2024. The study will consist of two groups of patients with peritoneal mesothelioma.
- Control group: This group will receive cisplatin plus Alimta® (pemetrexed). This combination is a standard systemic chemotherapy treatment for mesothelioma patients.
- Experimental group: This group will receive standard systemic chemotherapy plus PIPAC with cisplatin and doxorubicin.
The results from this and other PIPAC clinical trials will provide valuable information for the mesothelioma community.
Who Is Eligible for PIPAC Treatment?
PIPAC is an emerging treatment for peritoneal mesothelioma. Patients may qualify for PIPAC clinical trials. Each clinical trial will have its own unique eligibility criteria.
Early research suggests that PIPAC may be an option for patients ineligible for cytoreductive surgery (CRS). It is possible that PIPAC may help shrink tumors to the point where a patient becomes eligible for CRS. When combined with HIPEC, CRS can help improve some mesothelioma patients’ life expectancies.
Some patients may not be well enough to handle aggressive treatments. At least one study notes that PIPAC is not as painful or disabling as other chemotherapy techniques. This may make it an option for patients in need of gentler forms of treatment.
Mesothelioma patients interested in PIPAC should talk to their doctors. A mesothelioma doctor can help patients determine eligibility to receive PIPAC treatment. They can also help patients explore other treatment options.
Sources
City of Hope. PIPAC – Pressurized Intraperitoneal Aerosolized Chemotherapy.
ClinicalTrials.gov. Treatment of Malignant Peritoneal Mesothelioma (MESOTIP) (MESOTIP). Updated November 2022.
De Simone M, Vaira M, et al. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Oxaliplatin, Cisplatin, and Doxorubicin in Patients with Peritoneal Carcinomatosis: An Open-Label, Single-Arm, Phase II Clinical Trial. Biomedicines. May 2020;8(5):102. doi: 10.3390/biomedicines8050102
Dr. Jamie Murphy. Understanding the Difference Between PIPAC and HIPEC.
Girshally R, Demtröder C, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World Journal of Surgical Oncology. September 2016;14:253. doi: 10.1186/s12957-016-1008-0
Graversen M, Lundell L, et al. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) as an outpatient procedure. Pleura and Peritoneum. December 2018;3(4):2018. doi: 10.1515/pp-2018-0128
Kepenekian V, Péron J, et al. Non-resectable Malignant Peritoneal Mesothelioma Treated with Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) Plus Systemic Chemotherapy Could Lead to Secondary Complete Cytoreductive Surgery: A Cohort Study. Annals of Surgical Oncology. March 2022;29(3):2104-2113. doi: 10.1245/s10434-021-10983-2
Larbre V, Alyami M, et al. No Renal Toxicity After Repeated Treatment with Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in Patients with Unresectable Peritoneal Metastasis. Anticancer Research. December 2018;38(12):6869-6875. doi: 10.21873/anticanres.13062
Nadiradze G, Horvath P, et al. Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Cancers. January 2020;12(1):34. doi: 10.3390/cancers12010034
Odendahl K, Solass W, et al. Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). European Journal of Surgical Oncology. October 2015;41(10):1379-1385. doi: 10.1016/j.ejso.2015.06.001
Penn Medicine. Heated/Hyperthermic Intraperitoneal Chemotherapy (HIPEC).
Quénet Torrent Institute. Peritoneal carcinomatosis. What is the difference between the PIPAC and the HIPEC techniques? April 2020.
Raza A, Huang W, et al. Advances in the management of peritoneal mesothelioma. World Journal of Gastroenterology. September 2014;20(33):11700-11712. doi: 10.3748/wjg.v20.i33.11700
Sgarbura O, Gourgou S, et al. MESOTIP: Phase II multicenter randomized trial evaluating the association of PIPAC and systemic chemotherapy vs. systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma. Pleura and Peritoneum. July 2019;4(2):2019-0010. doi: 10.1515/pp-2019-0010
Sheba Global. PIPAC – Innovative, Direct Delivery of Chemotherapy for Peritoneal Metastasis.
Simkens GA, Rovers KP, et al. Patient selection for cytoreductive surgery and HIPEC for the treatment of peritoneal metastases from colorectal cancer. Cancer Management and Research. June 2017;9:259-266. doi: 10.2147/CMAR.S119569
Solass W, Kerb R, et al. Intraperitoneal Chemotherapy of Peritoneal Carcinomatosis Using Pressurized Aerosol as an Alternative to Liquid Solution: First Evidence for Efficacy. Annals of Surgical Oncology. February 2014;21(2):553-559. doi: 10.1245/s10434-013-3213-1
Tate SJ and Torkington J. Pressurized intraperitoneal aerosol chemotherapy: a review of the introduction of a new surgical technology using the IDEAL framework. BJS Open. April 2020;4(2):206-215. doi: 10.1002/bjs5.50257
Tufts Medical Center. HIPEC Surgery – What to Expect.
Willaert W, Sessink P, et al. Occupational safety of pressurized intraperitoneal aerosol chemotherapy (PIPAC). Pleura and Peritoneum. September 2017;2(3):121-128. doi: 10.1515/pp-2017-0018

Dr. Francis Perry Wilson is the Director of the Clinical and Translational Research Accelerator at the Yale University School of Medicine. He specializes in nephrology and clinical research.

Katy Moncivais, Ph.D., has more than 15 years of experience as a medical communicator. As the Medical Editor at Mesothelioma.com, she ensures our pages and posts present accurate, helpful information.

